Hydrochloric Acid: Applications, Safety, And Sourcing
Hydrochloric Acid: Applications, Safety, and Sourcing
Related Articles: Hydrochloric Acid: Applications, Safety, and Sourcing
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Hydrochloric Acid: Applications, Safety, and Sourcing. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Hydrochloric Acid: Applications, Safety, and Sourcing

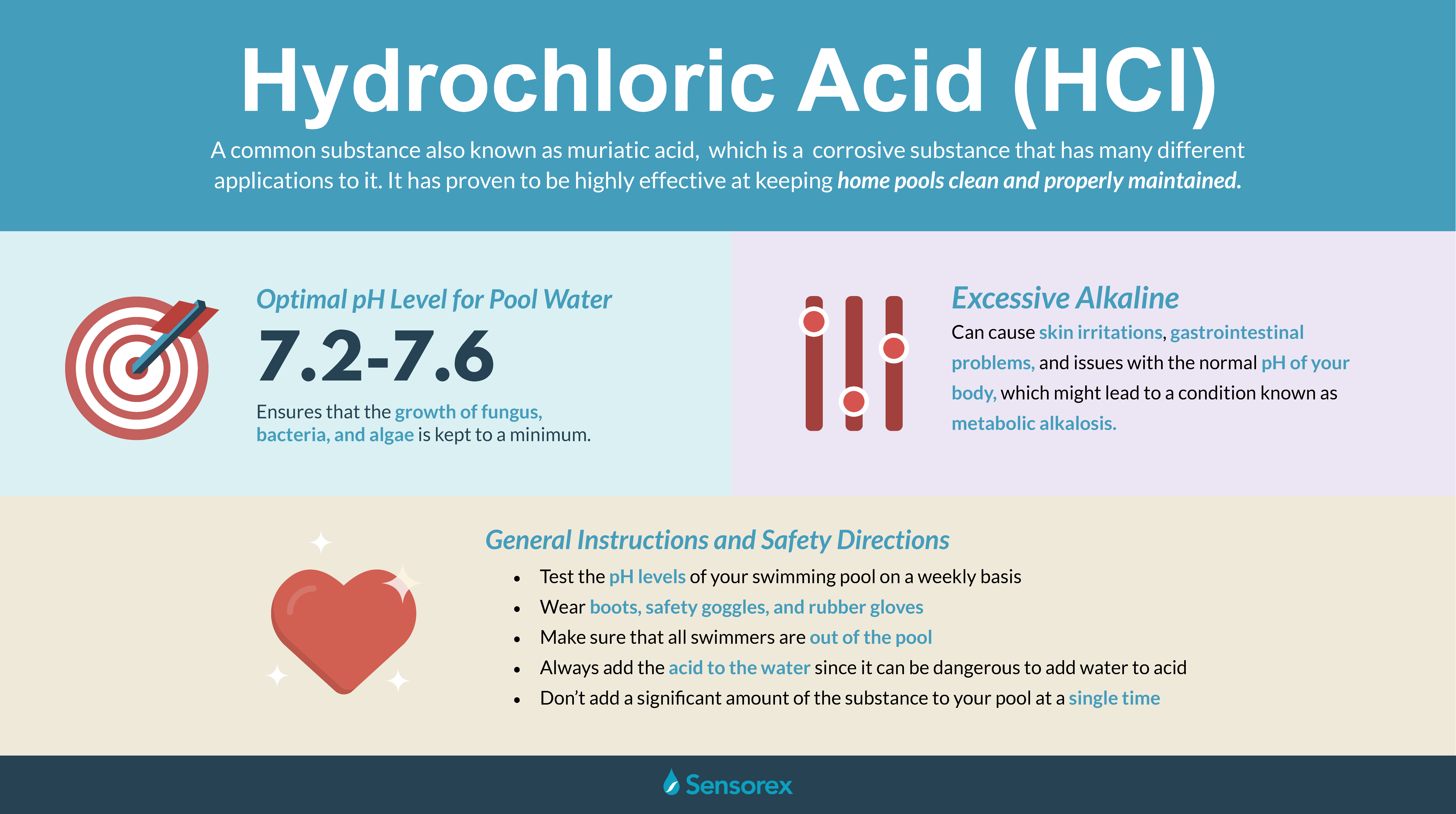
Hydrochloric acid (HCl), also known as muriatic acid, is a highly corrosive strong mineral acid with numerous industrial and commercial applications. Its importance stems from its ability to dissolve metals, oxides, and carbonates, making it a crucial component in various chemical processes and manufacturing activities.
Applications of Hydrochloric Acid:
The diverse applications of hydrochloric acid highlight its significance across multiple industries.
- Chemical Processing: Hydrochloric acid is extensively used in the production of various chemicals, including chlorides, fertilizers, and pharmaceuticals. It acts as a catalyst in several chemical reactions, facilitating the production of diverse products.
- Metal Processing: In the metal industry, hydrochloric acid plays a vital role in pickling, a process that removes oxides and other impurities from metal surfaces, preparing them for further processing or finishing. It is also used in the extraction of certain metals, like titanium and zirconium.
- Food Industry: Hydrochloric acid finds applications in the food industry as an acidity regulator, contributing to the desired taste and texture of various food products. It is also used in the production of gelatin and other food additives.
- Leather Tanning: Hydrochloric acid is employed in the leather tanning process, where it helps in removing unwanted hair and other impurities from animal hides, preparing them for the tanning process.
- Water Treatment: Hydrochloric acid is used to adjust the pH of water, ensuring optimal conditions for various processes like corrosion control and water purification.
- Construction: In the construction industry, hydrochloric acid is used to clean brickwork, remove mortar stains, and prepare surfaces for painting or other treatments.
- Laboratory Use: Hydrochloric acid is a common reagent in laboratories, utilized in various experiments and analytical procedures.
Safety Considerations with Hydrochloric Acid:
Hydrochloric acid is a hazardous substance requiring strict safety protocols during handling and storage.
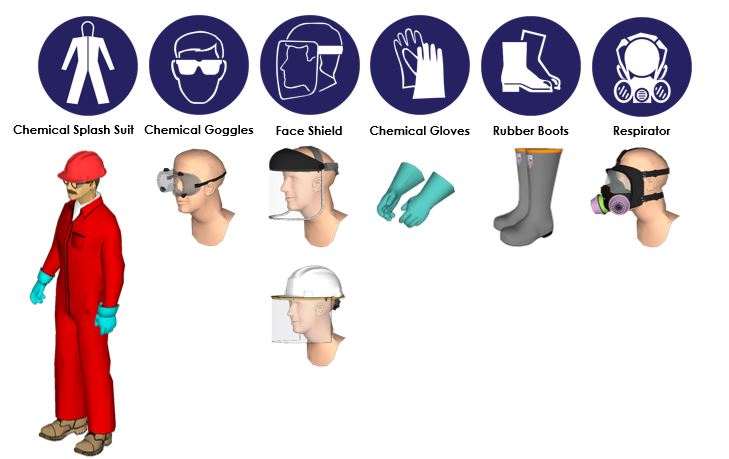
- Corrosive Nature: Its highly corrosive nature necessitates protective equipment, including gloves, goggles, and protective clothing, to prevent skin and eye contact.
- Inhalation Hazards: Inhalation of hydrochloric acid fumes can cause respiratory irritation, coughing, and even lung damage. Adequate ventilation is crucial during handling and storage.
- Mixing Hazards: Mixing hydrochloric acid with other chemicals, such as bleach, can generate toxic chlorine gas, highlighting the importance of careful handling and appropriate mixing procedures.
Sourcing Hydrochloric Acid:
Hydrochloric acid is readily available from various sources, including chemical suppliers, industrial distributors, and online retailers. When sourcing hydrochloric acid, it is crucial to consider the following factors:
- Purity and Concentration: The specific application will determine the required purity and concentration of hydrochloric acid.
- Quantity Requirements: The volume of hydrochloric acid needed will influence the choice of supplier and packaging options.
- Safety and Handling: Ensure the supplier adheres to strict safety regulations and provides appropriate safety data sheets (SDS) and handling instructions.
- Cost and Delivery: Compare prices and delivery options from different suppliers to find the most cost-effective solution.
FAQs about Hydrochloric Acid Sourcing:
Q: Where can I purchase hydrochloric acid?
A: Hydrochloric acid is available from various sources, including chemical suppliers, industrial distributors, and online retailers. It is essential to choose a reputable supplier with a proven track record of providing high-quality products and adhering to safety standards.
Q: What are the different grades of hydrochloric acid available?
A: Hydrochloric acid is available in various grades, ranging from technical grade to reagent grade. The grade will influence the purity and concentration of the acid, with reagent grade offering the highest purity and being suitable for analytical applications.
Q: What safety precautions should I take when handling hydrochloric acid?
A: Always handle hydrochloric acid with caution and wear appropriate protective equipment, including gloves, goggles, and protective clothing. Ensure adequate ventilation and avoid mixing it with other chemicals, particularly bleach.
Q: What are the legal requirements for purchasing hydrochloric acid?
A: The legal requirements for purchasing hydrochloric acid may vary depending on your location. It is advisable to consult local regulations and ensure compliance with all relevant laws and regulations.
Tips for Sourcing Hydrochloric Acid:
- Research and Compare Suppliers: Explore different suppliers, comparing their prices, product quality, safety records, and delivery options.
- Read Safety Data Sheets (SDS): Thoroughly review the SDS provided by the supplier to understand the hazards associated with hydrochloric acid and the necessary safety precautions.
- Consider Packaging Options: Choose the most appropriate packaging based on the quantity required, ensuring safe and convenient handling and storage.
- Confirm Delivery and Handling Procedures: Discuss delivery procedures and ensure the supplier adheres to safe handling practices during transportation and delivery.
- Store Properly: Store hydrochloric acid in a cool, dry, and well-ventilated area, away from incompatible materials.
Conclusion:
Hydrochloric acid is a versatile and powerful chemical with numerous applications across various industries. Its importance stems from its ability to dissolve metals, oxides, and carbonates, making it a crucial component in numerous chemical processes and manufacturing activities. However, its corrosive nature necessitates strict safety precautions during handling and storage. By carefully considering the factors discussed above, individuals and organizations can source hydrochloric acid responsibly, ensuring safe and efficient utilization for their specific needs.






Closure
Thus, we hope this article has provided valuable insights into Hydrochloric Acid: Applications, Safety, and Sourcing. We thank you for taking the time to read this article. See you in our next article!
You may also like
Recent Posts
- The Ubiquitous "T": A Journey Through Objects And Concepts
- Navigating The World Of Household Waste Removal: A Comprehensive Guide
- Navigating The Aftermath: A Comprehensive Guide To Post-Mortem Planning
- The Science Of Slime: A Guide To Creating Viscous Fun From Common Household Ingredients
- A Culinary Journey: Exploring Kitchen Household Items And Their Significance
- Navigating The Local Market: A Guide To Selling Household Items
- The Essentials Of Human Existence: A Comprehensive Look At The Items We Need
- The Intriguing World Of Six-Inch Objects: Exploring Everyday Items With A Specific Dimension
Leave a Reply